
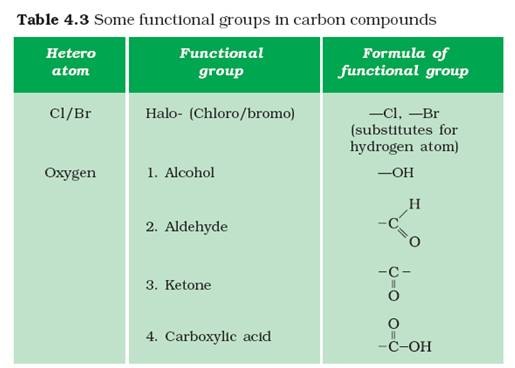
The second group is either a hydrogen or a carbon-based group. An aldehyde has at least one hydrogen connected to the carbonyl carbon. Both possess a carbonyl group, which is a carbon double bonded to an oxygen. A quick breakdown on how they are all related.Aldehydes and ketones have a similar structure. One more guest for the hydrogen bonding party. Naming ethers is beyond the scope of this chapter, however, this ether goes by the name diethyl ether. Naming is really getting complicated now. What combination is left? Ethers, lovers of oxygen linkages. For the record, this ester goes by the name methyl ethanoate. Good thing naming esters is beyond the scope of this chapter. What happens if the carboxyl group adds oxygen and it moves inside the carbon chain? We get esters. The majority of these reactions are only covered in upper level chemistry courses, but one, the infamous hydrogen bonding, is relevant to this chapter. The presence of a carboxylic acid on a hydrocarbon chain provides two oxygen atoms that can now participate in reactions. However, it received fame and fortune under its stage name: vinegar. This carboxylic acid has the proper name of ethanoic acid. These contain the carboxyl (not to be confused with the carbonyl group discussed above) functional group: -COOH. What happens when we see them all at the same time?Ĭarboxylic acids happen, that's what. We've seen double bonds, we've seen alcohols, and we've seen carbonyl groups. Naming aldehydes is beyond the scope of this chapter, but, to satisfy curiosity, this aldehyde is known as propanal.Īlso, The presence of an oxygen atom in an aldehyde group is another way for hydrocarbons to attend the hydrogen bonding party. If Aldehydes lived in New Orleans, their carbonyl group, which leads the hydrocarbon chain, could also be the Grand Marshall of the Mardi Gras parade.īoth Aldehydes and Grand Marshalls are leaders of hydrocarbons and marching bands, respectively. They also contain a carbonyl group, however this group is always found at the ends of the carbon chain, never inside of it. The presence of an oxygen atom in a ketone group allows the hydrocarbon to participate in the hydrogen bonding party.Īldehydes are the first cousin of ketones. The magic key that will let a carbonyl group into a hydrocarbon. Think of it this way, you need a "key" to get inside the hydrocarbon. The hydrocarbon cannot be on the first or last carbon of the chain. Unlike alcohols, ketones can only be found "inside" the hydrocarbon. Naming ketones is beyond the scope of this chapter, but, to satisfy curiosity, this ketone is known as 2-butanone. Carbonyl groups look similar to the hydroxyl groups found in alcohols, except they have a double bond with the oxygen. Double bonds are not exclusive to carbon atoms, however, as the carbonyl group, C=O, shows us. This alcohol goes by the name 1,2 butanol (with the 1 and 2 indicating the carbons the hydroxyl is attached to).Īlkenes introduced us to the double bond. The above alcohol goes by the name propanol. The hydroxyl group makes the hydrocarbon polar, allowing it to participate in hydrogen bonding.īoth polar bears and polar alcohols are able to mix with water through hydrogen bonding.Īlcohols are not above the naming rules, and they introduce us to the suffix - ol. The functional group that defines an alcohol is the hydroxyl group: –OH. Halogens are common functional groups for alkanes, and can be used in reactions to reduce an alkene into an alkane. Halogens are our friends from group 7 in the periodic table: fluorine (F), chlorine (Cl), bromine (Br), and iodine (I). Let's check them out them in more detail. These are the atoms involved in all the wheeling and dealing that causes electrons to shift around and for reactions to take place. Once we start adding ornaments, however, there is no telling how wild and crazy (reactive) the Christmas tree can get.įunctional groups are where the magic takes place. The tree itself is standard and pretty boring (non-reactive). Think of this in terms of a Christmas tree. When we first met alkanes early in this chapter, we learned that they could be thought of as the things that the more reactive portions (functional groups) of the molecule are attached to. Functional Groups Functional, Yet Stylish


 0 kommentar(er)
0 kommentar(er)
